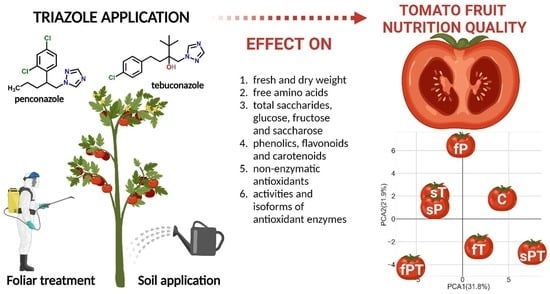
Triazoles as a Potential Threat to the Nutritional Quality of Tomato Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Fruit Harvest
2.2. Determination of Free Proteinogenic Amino Acids and Total Soluble Protein Content
2.3. Determination of Total Soluble and Membrane-Bound Saccharides
2.4. Determination of Glucose, Fructose and Saccharose
2.5. Total Phenolic and Flavonoid Contents and Antioxidant Capacity
2.6. Determination of Carotenoids and Chlorophylls
2.7. Identification of Secondary Metabolites
2.8. Antioxidant and Biotransformation Enzyme Activities
2.9. Statistical Analysis
3. Results
3.1. Physiological Parameters and Contents of Free Amino Acids and Proteins
3.2. Saccharide Contents
3.3. Total Phenolics, Flavonoids, Carotenoids and Antioxidant Capacity
3.4. Identification of Secondary Metabolites in Tomato Peel
3.5. Activites and Isoforms of Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Alseekh, S.; Fernie, A.R. On the regulation and function of secondary metabolism during fruit development and ripening. J. Exp. Bot. 2014, 65, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Nohara, T.; Ono, M.; Ikeda, T.; Fujiwara, Y.; El-Aasr, M. The tomato saponin, esculeoside A. J. Nat. Prod. 2010, 73, 1734–1741. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Bělonožníková, K.; Hýsková, V.; Chmelík, J.; Kavan, D.; Čeřovská, N.; Ryšlavá, H. Pythium oligandrum in plant protection and growth promotion: Secretion of hydrolytic enzymes, elicitors and tryptamine as auxin precursor. Microbiol. Res. 2022, 258, 126976. [Google Scholar] [CrossRef]
- Jakl, M.; Ćavar Zeljković, S.; Kovač, I.; Bělonožníková, K.; Jaklová Dytrtová, J. Side effects of triazoles on treated crops. Chemosphere 2021, 277, 130242. [Google Scholar] [CrossRef]
- Jakl, M.; Kovač, I.; Ćavar Zeljković, S.; Jaklová Dytrtová, J. Triazole fungicides in soil affect the yield of fruit, green biomass, and phenolics production of Solanum lycopersicum L. Food Chem. 2021, 351, 129328. [Google Scholar] [CrossRef]
- Egbuta, C.; Lo, J.; Ghosh, D. Mechanism of inhibition of estrogen biosynthesis by azole fungicides. Endocrinology 2014, 155, 4622–4628. [Google Scholar] [CrossRef]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 3. [Google Scholar] [CrossRef]
- Dvořák, Z. Drug-drug interactions by azole antifungals: Beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol. Lett. 2011, 202, 129–132. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Virus, C.; Waterman, M.R. Conservation in the CYP51 family. Role of the B’ helix/BC loop and helices F and G in enzymatic function. Biochemistry 2003, 42, 9091–9101. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Paradelo, M.; López, E.; Simal-Gándara, J. Influence of pH and soil copper on adsorption of metalaxyl and penconazole by the surface layer of vineyard soils. J. Agric. Food Chem. 2006, 54, 8155–8162. [Google Scholar] [CrossRef]
- Jakl, M.; Fanfrlík, J.; Jaklová Dytrtová, J. Mimicking of cyproconazole behavior in the presence of Cu and Zn. Rapid Commun. Mass Spectrom. 2017, 31, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Jaklová Dytrtová, J.; Bělonožníková, K.; Jakl, M.; Ryšlavá, H. Triazoles and aromatase: The impact of copper cocktails. Environ. Pollut. 2020, 266, 115201. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Jakl, M.; Schröder, D.; Čadková, E.; Komárek, M. Complexation between the fungicide tebuconazole and copper(II) probed by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1037–1042. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Straka, M.; Bělonožníková, K.; Jakl, M.; Ryšlavá, H. Does resveratrol retain its antioxidative properties in wine? Redox behaviour of resveratrol in the presence of Cu(II) and tebuconazole. Food Chem. 2018, 262, 221–225. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Bělonožníková, K.; Jakl, M.; Chmelík, J.; Kovač, I.; Ryšlavá, H. Non-target biotransformation enzymes as a target for triazole-zinc mixtures. Chem. Biol. Interact. 2023, 382, 110625. [Google Scholar] [CrossRef] [PubMed]
- Valadas, J.; Mocelin, R.; Sachett, A.; Marcon, M.; Zanette, R.A.; Dallegrave, E.; Herrmann, A.P.; Piato, A. Propiconazole induces abnormal behavior and oxidative stress in zebrafish. Environ. Sci. Pollut. Res. Int. 2019, 26, 27808–27815. [Google Scholar] [CrossRef]
- Mallano, A.I.; Yu, J.; Dina, T.; Li, F.; Ling, T.; Ahmad, N.; Bennetzen, J.; Tong, W. Soil and fine root-associated microbial communities are niche dependent and inf luenced by copper fungicide treatment during tea plant cultivation. Hortic. Res. 2023, 10, uhac285. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A comprehensive review on environmental toxicity of azole compounds to fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef]
- Casida, J.E. Pest toxicology: The primary mechanisms of pesticide action. Chem. Res. Toxicol. 2009, 22, 609–919. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-F.; Ying, G.-G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef]
- Schmidt, F.; Marx-Stoelting, P.; Haider, W.; Heise, T.; Kneuer, C.; Ladwig, M.; Banneke, S.; Rieke, S.; Niemann, L. Combination effects of azole fungicides in male rats in a broad dose range. Toxicology 2016, 355, 54–63. [Google Scholar] [CrossRef]
- Cao, F.; Souders, I.C.L.; Li, P.; Pang, S.; Qiu, L.; Martyniuk, C.J. Developmental toxicity of the triazole fungicide cyproconazole in embryo-larval stages of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 4913–4923. [Google Scholar] [CrossRef]
- Li, S.; Sun, Q.; Wu, Q.; Gui, W.; Zhu, G.; Schlenk, D. Endocrine disrupting effects of tebuconazole on different life stages of zebrafish (Danio rerio). Environ. Pollut. 2019, 249, 1049–1059. [Google Scholar] [CrossRef]
- Taxvig, C.; Vinggaard, A.M.; Hass, U.; Axelstad, M.; Metzdorff, S.; Nellemann, C. Endocrine-disrupting properties in vivo of widely used azole fungicides. Int. J. Androl. 2008, 31, 170–176. [Google Scholar] [CrossRef]
- Trösken, E.R.; Fischer, K.; Völkel, W.; Lutz, W.K. Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 2006, 219, 33–40. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Vatsa, P.; Clement, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Gilley, A.; Sankhla, N.; Davis, T.M. Triazoles as plant growth regulators and stress protectants. Hort. Rev. 2000, 24, 56–138. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Panneerselvam, R. Growth and photosynthetic pigments responses of two varieties of Catharanthus roseus to triadimefon treatment. C. R. Biol. 2008, 331, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, R.; Zheng, M.; Cai, C.; Diao, J.; Zhou, Z. Hexaconazole application saves the loss of grey mold disease but hinders tomato fruit ripening in healthy plants. J. Agric. Food Chem. 2022, 70, 3948–3957. [Google Scholar] [CrossRef]
- Kovač, I.; Jakl, M.; Šolínová, V.; Konášová, R.; Kašička, V.; Jaklová Dytrtová, J. Micellar electrokinetic chromatography in the determination of triazoles in fruit peel. J. Chromatogr. A 2021, 1652, 462385. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Simal-Gandara, J.; Tian, J.; Chen, J.; Dai, X.; Kong, Z. Impact of chiral tebuconazole on the flavor components and color attributes of Merlot and Cabernet Sauvignon wines at the enantiomeric level. Food Chem. 2022, 373, 131577. [Google Scholar] [CrossRef]
- Xiao, O.; Li, M.; Chen, J.; Li, R.; Quan, R.; Zhang, Z.; Kong, Z.; Dai, X. Influence of triazole pesticides on wine flavor and quality based on multidimensional analysis technology. Molecules 2020, 25, 5596. [Google Scholar] [CrossRef] [PubMed]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal agents in agriculture: Friends and foes of public health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, B.D.; White, T.D. Accumulation of azole drugs in the fungal plant pathogen Magnaporthe oryzae is the result of facilitated diffusion influx. Front. Microbiol. 2017, 8, 1320. [Google Scholar] [CrossRef]
- Hodek, O.; Krizek, T.; Coufal, P.; Ryslava, H. Design of experiments for amino acid extraction from tobacco leaves and their subsequent determination by capillary zone electrophoresis. Anal. Bioanal. Chem. 2017, 409, 2383–2391. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hýsková, V.; Bělonožníková, K.; Doričová, V.; Kavan, D.; Gillarová, S.; Henke, S.; Synková, H.; Ryšlavá, H.; Čeřovská, N. Effects of heat treatment on metabolism of tobacco plants infected with Potato virus Y. Plant Biol. J. 2021, 23, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Tomníková, A.; Kozlík, P.; Křížek, T. Monosaccharide profiling of glycoproteins by capillary electrophoresis with contactless conductivity detection. Electrophoresis 2022, 43, 1963–1970. [Google Scholar] [CrossRef]
- Tupec, M.; Hýsková, V.; Bělonožníková, K.; Hraníček, J.; Červený, V.; Ryšlavá, H. Characterization of some potential medicinal plants from Central Europe by their antioxidant capacity and the presence of metal elements. Food Biosci. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Ryšlavá, H.; Pomeislová, A.; Pšondrová, S.; Hýsková, V.; Smrček, S. Phytoremediation of carbamazepine and its metabolite 10,11-epoxycarbamazepine by C3 and C4 plants. Environ. Sci. Pollut. Res. 2015, 22, 20271–20282. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Effect of patulin from Penicillium vulpinum on the activity of glutathione-S-transferase and selected antioxidative enzymes in maize. Int. J. Environ. Res. Public Health 2017, 14, 825. [Google Scholar] [CrossRef]
- Spoustová, P.; Hýsková, V.; Müller, K.; Schnablová, R.; Ryšlavá, H.; Čeřovská, N.; Malbeck, J.; Cvikrová, M.; Synková, H. Tobacco susceptibility to Potato virus Y-NTN infection is affected by grafting and endogenous cytokinin content. Plant Sci. 2015, 235, 25–36. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Detection of ascorbate peroxidase-activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 1993, 212, 540–546. [Google Scholar] [CrossRef]
- Synková, H.; Semoradová, S.; Schnablová, R.; Müller, K.; Pospíšilová, J.; Ryšlavá, H.; Malbeck, J.; Čeřovská, N. Effects of biotic stress caused by Potato virus Y on photosynthesis in ipt transgenic and control Nicotiana tabacum L. Plant Sci. 2006, 171, 607–616. [Google Scholar] [CrossRef]
- Lin, C.L.; Chen, H.J.; Hou, W.C. Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecyl sulfate polyacrylamide gels. Electrophoresis 2002, 23, 513–516. [Google Scholar] [CrossRef]
- Ferguson, K.A. Starch-gel electrophoresis—Application to the classification of pituitary proteins and polypeptides. Metabolism 1964, 13, 985–1002. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A comprehensive evaluation of tomato fruit quality and identification of volatile compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.M.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Dixon, G.R. Amino acid changes during the early stages of tomato wilt disease (Verticillium albo-atrum). Plant Prot. Sci. 2021, 57, 140–147. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, S.; Sun, T.; Zhou, Z.; Hu, Z.; Yu, J. Rosmarinic acid delays tomato fruit ripening by regulating ripening-associated traits. Antioxidants 2021, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Deng, Y.; Zhou, Y.; Liu, R.; Liu, Y.; Wang, H.; Zhu, W.; Zhou, Z.; Diao, J. Multifaceted effects of difenoconazole in tomato fruit ripening: Physiology, flavour and nutritional quality. Plant Physiol. Biochem. 2023, 194, 223–235. [Google Scholar] [CrossRef]
- Dai, Z.; Zheng, W.; Locasale, J.W. Amino acid variability, tradeoffs and optimality in human diet. Nat. Commun. 2022, 13, 6683. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.M.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 2020, 586, 790–795. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Chen, H.C.; Li, Q.; Shuford, C.M.; Liu, J.; Muddiman, D.C.; Sederoff, R.R.; Chiang, V.L. Membrane protein complexes catalyze both 4- and 3-hydroxylation of cinnamic acid derivatives in monolignol biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 21253–21258. [Google Scholar] [CrossRef]
- Swayambhu, G.; Raghavan, I.; Ravi, B.G.; Pfeifer, B.A.; Wang, Z.Q. Salicylate glucoside as a nontoxic plant protectant alternative to salicylic acid. ACS Agric. Sci. Technol. 2021, 1, 515–521. [Google Scholar] [CrossRef]
- Feng, K.; Yu, J.; Cheng, Y.; Ruan, M.; Wang, R.; Ye, Q.; Zhou, G.; Li, Z.; Yao, Z.; Yang, Y.; et al. The SOD gene family in tomato: Identification, phylogenetic relationships, and expression patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef]
- Rabert, G.A.; Manivannan, P.; Somasundaram, R.; Panneerselvam, R. Triazole compounds alters the antioxidant and osmoprotectant status in drought stressed Helianthus annuus L. plants. Emir. J. Food Agric. 2014, 26, 265–276. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Feng, N.; Xiang, H.; Liu, Y.; Wang, F.; Li, W.; Feng, S.; Liu, M.; Zheng, D. Physiological response of soybean leaves to uniconazole under waterlogging stress at R1 stage. J. Plant Physiol. 2022, 268, 153579. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Shaki, F.; Niknam, V.; Ebrahimzadeh Maboud, H. Different effects of penconazole on enzymatic and non-enzymatic antioxidants of sesame (Sesamum indicum L.) under salinity. Mod. Concepts Dev. Agron. 2019, 4, 467–473. [Google Scholar] [CrossRef]
- Tuna, A.L. Influence of foliarly applied different triazole compounds on growth, nutrition, and antioxidant enzyme activities in tomato (Solanum lycopersicum L.) under salt stress. Aust. J. Crop Sci. 2014, 8, 71–79. [Google Scholar]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef] [PubMed]
- Arsand, D.R.; Soares da Cunha, S.; Fuentes-Guevara, M.D.; Ramires Araujo, T.; Gilberto Primel, E.; Caldas Barbosa, S.; Érico Kunde Corrêa, E. Photodegradation of tebuconazole in aqueous solution and phytotoxic effects. Environ. Eng. Manag. J. 2021, 20, 1535–1542. Available online: https://eemj.eu/index.php/EEMJ/article/view/4393 (accessed on 25 July 2023).
- Zhang, X.; Wang, X.; Luo, F.; Sheng, H.; Zhou, L.; Zhong, Q.; Lou, Z.; Sun, H.; Yang, M.; Cui, X.; et al. Application and enantioselective residue determination of chiral pesticide penconazole in grape, tea, aquatic vegetables and soil by ultra performance liquid chromatography-tandem mass spectrometry. Ecotox. Environ. Saf. 2019, 172, 530–537. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hýsková, V.; Jakl, M.; Jaklová Dytrtová, J.; Ćavar Zeljković, S.; Vrobel, O.; Bělonožníková, K.; Kavan, D.; Křížek, T.; Šimonová, A.; Vašková, M.; et al. Triazoles as a Potential Threat to the Nutritional Quality of Tomato Fruits. Metabolites 2023, 13, 988. https://doi.org/10.3390/metabo13090988
Hýsková V, Jakl M, Jaklová Dytrtová J, Ćavar Zeljković S, Vrobel O, Bělonožníková K, Kavan D, Křížek T, Šimonová A, Vašková M, et al. Triazoles as a Potential Threat to the Nutritional Quality of Tomato Fruits. Metabolites. 2023; 13(9):988. https://doi.org/10.3390/metabo13090988
Chicago/Turabian StyleHýsková, Veronika, Michal Jakl, Jana Jaklová Dytrtová, Sanja Ćavar Zeljković, Ondřej Vrobel, Kateřina Bělonožníková, Daniel Kavan, Tomáš Křížek, Alice Šimonová, Marie Vašková, and et al. 2023. "Triazoles as a Potential Threat to the Nutritional Quality of Tomato Fruits" Metabolites 13, no. 9: 988. https://doi.org/10.3390/metabo13090988


.png)